There’s a lot of new age misdirection surrounding pH balance. An entire sub-economy of foods and beverages populated the marketplace seemingly overnight to fight off the woes of every ailment the human body faces.
Brands can throw around statements like, “acidosis is associated with cancer” to make way for a pitch about their brand of alkaline water. There’s a nugget of truth buried deep in there, but there has been no demonstrable causal link between chronic acidosis and proliferation of cancer <1>. The effects of a chronically acidic diet are pervasive and aren’t entirely within the scope of our understanding. We are, however, aware that an acidic environment can curtail sustained athletic performance, and maintaining a “balanced” pH is conducive to superior results. But before we dive into that mechanism, let’s do a bit of basic chemistry refreshing.
What is pH balance? Why do I care? pH stands for Potential of Hydrogen and determines the acidity or alkalinity of a substance. pH is measured on scale of 0 to 14, with acids comprising the 0 to 7 mark and bases filling out the scale from 7 to 14. Generally, every liquid that isn’t water we imbibe is somewhat acidic. Even milk, which is very close to neutral, has a pH balance of 6.6.
Acids are substances that can donate hydrogen molecules to another substance as needed, whereas bases will accept hydrogen molecules from substances. This mechanism is important when we discuss athletic performance shortly.
Pure water is the only substance we ingest that has a truly “balanced” pH of 7, being neither alkaline nor acidic. Most water we actually ingest daily can range from slightly acidic to slightly alkaline, but hovering close to this mark of 7 on th pH scale.
It should come as no surprise that the human body, largely comprised of water, maintains a pH level between 7.35 to 7.45 <2>. Disturbing this equilibrium is something the body will not tolerate, and it will deploy any number of self-protection mechanisms to maintain it, such as “the burn” during exercise induced stress. Acidosis is the condition of lower pH value than optimal and can be brought on by any number of diet, exertion, and stress related factors.
How do I maintain a pH balance? Your body does a pretty wonderful job of keeping pH balance to ensure its own survival, however, daily diet and routine determine whether or not your body has the necessary tools to maintain its homeostasis. Unfortunately, a lot of the food and beverages ingested in western culture tend to be more acidic and lacking the balance necessary to help the body stay healthy. Most meats, dairy products, grains, and protein supplements are acidic by nature. This does not make them bad foods, it is simply their chemical composition. Add beverages like coffee, tea, sports drinks, juice, or virtually everything but pure water, and nearly all the things ingested on a daily basis are at least somewhat acidic. When thrust into a constant state of acidosis, the human body has a difficult time maintaining homeostasis, and over time may lead to degenerative diseases in the bones, kidneys, and other areas.
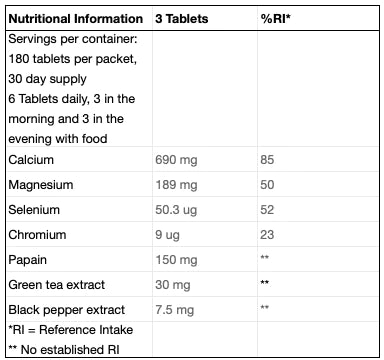
Chronic acidosis can be prevented by eating base or alkaline substances rich with the salts magnesium, calcium, and potassium. What magical source can we possibly find theses bases you ask? In fruits and vegetables. Turns out the conventional wisdom of eat more vegetables was right for a reason. Common alkaline minerals like calcium carbonate or magnesium hydroxide have pH values ranging between 9 and 11. With proliferation of processed foods and increased protein ingestion, our bodies need more fruits and vegetables than ever before. These minerals found within act as the buffers towards acidic substances and support the body’s optimal state of being.
Ok, but what about athletes? Those who train vigorously and regularly expend a great deal more calories than the average population. Greater caloric demands mean a larger volume of food necessary to recover and continue fueling training and increased demands on your body’s ability to maintain homeostasis. While most successful athletes will include a robust amount of fruits and vegetables in their diet, the stress of exertion during training may outweigh what they can logistically replenish.
Athletes are especially sensitive to acidosis during stressful exercise because of the hydrogen ion mechanism we spoke about earlier. In anaerobic training, large amounts of lactic acid, or lactate and a hydrogen ion, are produced. Lactate itself is not responsible for the painful “burning” soreness sensation, and is actually the substance providing the body with fuel to sustain a prolonged difficult exertion. However, an environment with an excess of hydrogen ions will turn acidic and lead to the “burning” response most feel. This is a self-protection mechanism, the body’s way of informing you that this level of effort may compromise your pH balance and homeostasis, thus it cannot be sustained.
Enter the Xendurance Lactic Acid Buffer
Alkaline substances, like the calcium, magnesium, and potassium found in Xendurance can act as a buffer against this acidic environment, delaying or preventing the body’s attempts to shut down intense training. Supplementing with Xendurance ensures the body has the tools it needs to support an optimal pH balance in the face of a typically acidic diet and exercise induced lactic acidosis. This means shorter recovery times, prolonged ability to train, and better results.
How do we know Xendurance can do this? Xendurance has spent the last decade putting it to the test through multiple, independent clinical trials. The most recent study in 2016 at the University of Lousiana-Lafayette, demonstrated a 26% reduction in lactic acid, 39% reduction in oxidative stress, and a six time reduction in creatine kinase (CK) levels, the marker associated with muscular damage <3>.
You can fuel performance, ensure long term health, and fight off degenerative diseases just by ensuring an your body has the tools it needs for optimal pH balance. Eat fruits and vegetables and give it the extra support it needs with daily Xendurance.
References:
Robbey, Ian Forresst. Examining the relationship between diet-induced acidosis and cancer. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3571898/. Waugh A, Grant A. Anatomy and Physiology in Health and Illness. 10th edition. Philadelphia, Pa, USA: Churchill Livingstone Elsevier; 2007. Bellar, David. The Effects of 10 Days of Extreme Endurance on Performance, Buffering Capacity During Exercise and Post Exercise Muscle Damage, Oxidative Stress and Inflammation.